Celiac Disease Clinical Trial Pipeline Gains Momentum: 20+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight
Celiac disease represents a growing global health concern, with current management limited to lifelong adherence to a gluten-free diet—an approach often associated with compliance challenges and reduced quality of life. The increasing prevalence of diagnosed cases and significant unmet clinical needs are accelerating demand for alternative therapeutic solutions.
New York, USA, Dec. 10, 2025 (GLOBE NEWSWIRE) -- Celiac Disease Clinical Trial Pipeline Gains Momentum: 20+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight
Celiac disease represents a growing global health concern, with current management limited to lifelong adherence to a gluten-free diet—an approach often associated with compliance challenges and reduced quality of life. The increasing prevalence of diagnosed cases and significant unmet clinical needs are accelerating demand for alternative therapeutic solutions.
DelveInsight’s 'Celiac Disease Pipeline Insight 2025' report provides comprehensive global coverage of pipeline therapies for celiac disease across various stages of clinical development. The report offers an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance the pipeline and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the celiac disease domain.
Celiac Disease Clinical Trial Analysis Summary
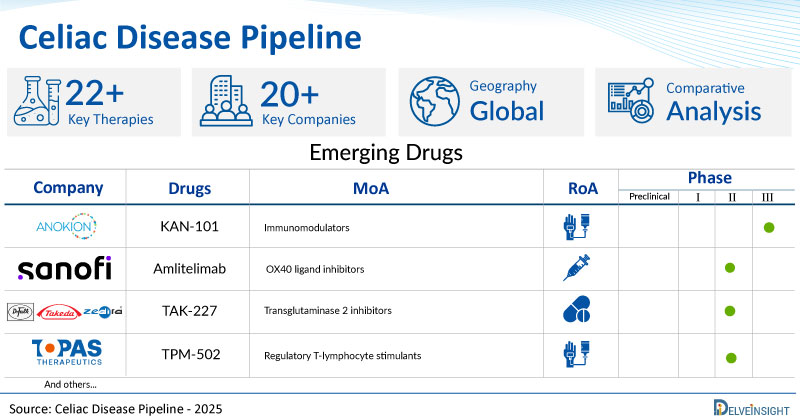
- DelveInsight’s celiac disease pipeline report depicts a robust space with 20+ active players working to develop 22+ pipeline celiac disease drugs.
- Key celiac disease companies such as Sanofi, Takeda, Barinthus Biotherapeutics, Topas Therapeutics, Anokion SA, Equillium, Forte Biosciences, Teva Pharmaceutical, Ahead Therapeutics, IGY Life Sciences, Mozart Therapeutics, Allero Therapeutics, Immunic Therapeutics, Chugai Pharmaceutical, and others are evaluating new celiac disease drugs to improve the treatment landscape.
- Promising pipeline celiac disease therapies, such as Amlitelimab, TAK-227, VTP-1000, TPM-502, KAN 101, EQ 302, FB 102, TEV-53408, AT 1718, IgY-112, MTX-101, ALL-001, IMU-856, DONQ52, and others, are in different phases of celiac disease clinical trials.
- Approximately 5+ celiac disease drugs are in the mid stage of development, whereas 14+ drugs are in the early stages of development.
- Notable MoAs in celiac disease clinical trials include OX40 ligand inhibitors, Transglutaminase 2 inhibitors, Regulatory T-lymphocyte stimulants, Immunostimulants, Interleukin 15 inhibitors; Interleukin 21 inhibitors, and others.
Request a sample and discover the recent advances in celiac disease drugs @ Celiac Disease Pipeline Report
What is Celiac Disease?
Celiac disease, also known as gluten-sensitive enteropathy, is an autoimmune disorder that affects the small intestine. In this condition, the body mounts an abnormal immune response to gluten, resulting in inflammation and damage to the small intestinal lining. Although first described by Samuel Gee in 1888, it was only in 1953 that gluten was identified as the key factor in its development. Celiac disease (CeD) can be considered a syndrome due to its diverse clinical manifestations and involvement of multiple body systems. Triggered by gluten ingestion, it produces a wide range of symptoms that vary greatly among individuals. Common gastrointestinal symptoms include chronic diarrhea, abdominal pain, bloating, constipation, and nausea, often accompanied by pale, foul-smelling stools. In addition to digestive issues, patients frequently experience non-digestive symptoms such as fatigue, anemia, joint pain, and neurological problems like headaches or numbness. In children, celiac disease may also present with delayed growth and mood disturbances.
Find out more about celiac disease drugs @ Celiac Disease Treatment
A snapshot of the Pipeline Celiac Disease Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| KAN-101 | AnokionSA | II | Immunomodulators | Intravenous |
| Amlitelimab | Sanofi | II | OX40 ligand inhibitors | Subcutaneous |
| TAK-227 | Dr Falk Pharma/Takeda/ Zedira | II | Transglutaminase 2 inhibitors | Oral |
| TPM-502 | Topas Therapeutics | II | Regulatory T-lymphocyte stimulants | Intravenous |
| VTP-1000 | Barinthus Biotherapeutics | I | Immunostimulants | Intramuscular |
| EQ 302 | Equillium | Preclinical | Interleukin 15 inhibitors; Interleukin 21 inhibitors | Oral |
Learn more about the emerging celiac disease therapies @ Celiac Disease Clinical Trials 2025
Recent Developments in Celiac Disease Treatment Space
- In June 2025, Forte Biosciences, Inc. announced positive data from a Phase Ib trial in celiac disease for lead program FB102 (FB102-101).
- In May 2025, Topas Therapeutics presented positive clinical proof-of-concept data demonstrating gluten-specific tolerance induction in celiac disease (CeD) patients. Data from a Phase IIa clinical trial evaluating TPM502 demonstrated a positive safety and tolerability profile, a significant reduction of the inflammatory responses to gluten and long-lasting phenotypic changes to gliadin-specific T cells.
- In May 2025, Teva Pharmaceutical Industries, Ltd. announced that the US Food and Drug Administration (FDA) granted Fast Track designation for investigational TEV-53408 for the treatment of people with celiac disease on a gluten-free diet.
- In March 2025, Mozart Therapeutics announced the successful completion of their Phase Ia study of MTX-101 in healthy adults.
- In January 2025, Anokion SA announced positive symptom data from its Phase II ACeD-it trial evaluating its lead candidate, KAN-101, in individuals with celiac disease.
- In September 2024, Barinthus Biotherapeutics announced the initiation of its first-in-human Phase I trial of VTP-1000 in adults with celiac disease.
Scope of the Celiac Disease Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: OX40 ligand inhibitors, Transglutaminase 2 inhibitors, Regulatory T-lymphocyte stimulants, Immunostimulants, Interleukin 15 inhibitors; Interleukin 21 inhibitors
- Key Celiac Disease Companies: Sanofi, Takeda, Barinthus Biotherapeutics, Topas Therapeutics, Anokion SA, Equillium, Forte Biosciences, Teva Pharmaceutical, Ahead Therapeutics, IGY Life Sciences, Mozart Therapeutics, Allero Therapeutics, Immunic Therapeutics, Chugai Pharmaceutical and others.
- Key Celiac Disease Pipeline Therapies: Amlitelimab, TAK-227, VTP-1000, TPM-502, KAN 101, EQ 302, FB 102, TEV-53408, AT 1718, IgY-112, MTX-101, ALL-001, IMU-856, DONQ52 and others.
Dive deep into rich insights for new celiac disease treatments, visit @ Celiac Disease Drugs
Table of Contents
| 1. | Celiac Disease Pipeline Report Introduction |
| 2. | Celiac Disease Pipeline Report Executive Summary |
| 3. | Celiac Disease Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Celiac Disease Clinical Trial Therapeutics |
| 6. | Celiac Disease Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Celiac Disease Pipeline: Late-Stage Products (Phase III) |
| 8. | Celiac Disease Pipeline: Mid-Stage Products (Phase II) |
| 9. | Celiac Disease Pipeline: Early-Stage Products (Phase I) |
| 10. | Celiac Disease Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Celiac Disease Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Celiac Disease Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the celiac disease cure research 2025, reach out @ Medication for Celiac Disease Treatment
Related Reports
Celiac Disease Epidemiology Forecast
Celiac Disease Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted Celiac Disease epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Celiac Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key celiac disease companies, including 9 Meters Biopharma, ImmunogenX, Provention Bio, Takeda, Cour Pharmaceuticals, Precigen ActoBio, Falk Pharma, Zedira, among others.
Sjogren's Syndrome Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Sjogren's syndrome companies including Horizon Therapeutics (Amgen), Dompe Farmaceutici, Sylentis, MorphoSys, Resolve Therapeutics, OSE Immunotherapeutics, Servier, Novartis, Johnson & Johnson, among others.
Sjogren's Syndrome Clinical Trial Analysis
Sjogren's Syndrome Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Sjogren's syndrome companies, including Novartis, Horizon Therapeutics, Bristol-Myers Squibb, Rise Therapeutics, Resolve Therapeutics, Dompe Farmaceutici, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
