Niemann-Pick Disease Type C Market is Expected to Showcase Significant Growth at a CAGR of 14.8% During the Forecast Period (2025–2034) | DelveInsight
The Niemann-Pick disease type C market is limited by a few approved treatments and a high unmet need. However, the launch of emerging therapies, such as Trappsol Cyclo by Cyclo Therapeutics, oral small-molecule candidate nizubaglustat (AZ-3102) by Azafaros, and early-stage candidate Adrabetadex (VTS-270), along with rising awareness, are expected to drive modest growth and improve future therapeutic options.
New York, USA, Oct. 29, 2025 (GLOBE NEWSWIRE) -- Niemann-Pick Disease Type C Market is Expected to Showcase Significant Growth at a CAGR of 14.8% During the Forecast Period (2025–2034) | DelveInsight
The Niemann-Pick disease type C market is limited by a few approved treatments and a high unmet need. However, the launch of emerging therapies, such as Trappsol Cyclo by Cyclo Therapeutics, oral small-molecule candidate nizubaglustat (AZ-3102) by Azafaros, and early-stage candidate Adrabetadex (VTS-270), along with rising awareness, are expected to drive modest growth and improve future therapeutic options.
DelveInsight’s Niemann-Pick Disease Type C Market Insights report includes a comprehensive understanding of current treatment practices, emerging Niemann-Pick disease type C drugs, market share of individual therapies, and current and forecasted Niemann-Pick disease type C market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
Niemann-Pick Disease Type C Market Summary
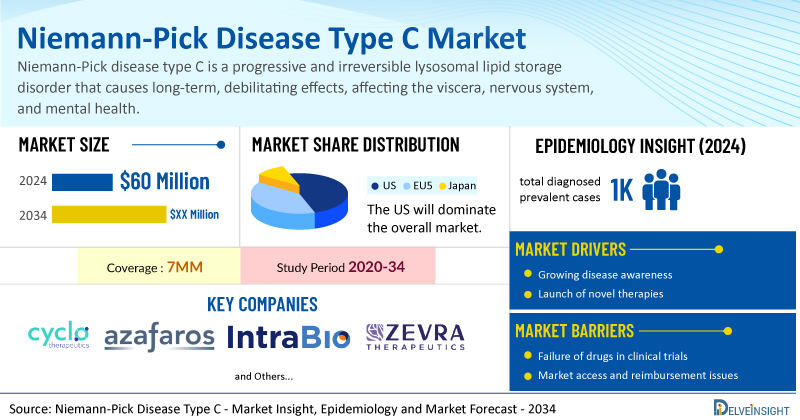
- The market size for Niemann-Pick disease type C was found to be USD 60 million in the leading markets in 2024.
- The United States accounted for the largest Niemann-Pick disease type C treatment market size, approximately 60% of the total market size in the 7MM in 2024, compared to other major markets, including the EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- In 2024, the total diagnosed prevalent cases of Niemann-Pick disease type C were approximately 1000 in the 7MM, highlighting its ultra-rare nature.
- Key Niemann-Pick disease type C companies, including Cyclo Therapeutics, Azafaros, Mandos, and others, are actively working on innovative Niemann-Pick disease type C drugs.
- Some of the key Niemann-Pick disease type C therapies in clinical trials include Trappsol Cyclo (hydroxypropyl-beta-cyclodextrin), Nizubaglustat (AZ-3102), Adrabetadex (VTS-270), and others. These novel Niemann-Pick disease type C therapies are anticipated to enter the Niemann-Pick disease type C market in the forecast period and are expected to change the market.
Discover which Niemann-Pick disease type C medications are expected to grab the market share @ Niemann-Pick Disease Type C Market Report
Key Factors Driving the Growth of the Niemann-Pick Disease Type C Market
Rising NPC Prevalence
According to the analysis conducted by DelveInsight, in 2024, the total diagnosed prevalent cases of NPC in the 7MM were nearly 1000. These cases are projected to increase during the forecast period (2025–2034).
Advancements in Genetic Research and Gene Therapy
Recent breakthroughs in understanding the genetic mutations responsible for NPC, particularly in the NPC1 and NPC2 genes, have paved the way for targeted therapies. Gene therapies, including gene replacement and editing techniques like CRISPR-Cas9, are emerging as promising treatments, offering potential curative options for this rare disorder.
Emergence of Novel NPC Therapies
The NPC therapeutic pipeline includes Trappsol Cyclo by Cyclo Therapeutics, oral small-molecule candidate nizubaglustat (AZ-3102) by Azafaros, and Mandos’ early-stage candidate Adrabetadex (VTS-270), reflecting a diversified approach targeting distinct disease mechanisms.
Development of Cyclodextrin-Based Therapies
Cyclodextrin-based therapies, such as Trappsol Cyclo, aim to remove cholesterol accumulations from cells, restoring normal cellular function. These therapies are gaining traction as they address the underlying lipid storage issues in NPC, contributing to NPC market growth.
Niemann-Pick Disease Type C Market Analysis
Current management of NPC is primarily symptom-focused and tailored to each patient. Difficulty swallowing should be closely monitored due to the risk of aspiration. Early interventions may include softening solid foods and thickening liquids, while speech therapists can help optimize swallowing techniques. In some cases, a gastrostomy tube may become necessary to ensure sufficient caloric intake. This involves inserting a thin tube directly into the stomach through a small abdominal incision, enabling direct delivery of nutrition or medication.
At present, two therapies, AQNEURSA (levacetylleucine) and MIPLYFFA (arimoclomol), are available for managing neurological symptoms of NPC, each with specific patient eligibility criteria. MIPLYFFA became the first FDA-approved oral treatment for this ultra-rare disease in September 2024, indicated for use alongside miglustat in patients aged two and older, a significant advance in symptomatic management. In July 2025, Zevra Therapeutics filed a Marketing Authorization Application (MAA) with the European Medicines Agency (EMA) for arimoclomol, targeting the underlying lipid accumulation in NPC.
Despite these options, no therapy has been proven to alter the underlying disease course. Current treatments address symptoms rather than providing a cure or disease-modifying effect. Miglustat remains important, approved in Europe (2009) and Japan (2012) to reduce symptoms and slow neurodegeneration, and is often used off-label in the U.S., reflecting a reliance on its symptomatic benefits in the absence of broader treatment alternatives. This underscores a significant unmet need for therapies that can modify NPC progression.
Learn more about the NPC treatment options @ Niemann-Pick Disease Type C Treatment Market
Niemann-Pick Disease Type C Competitive Landscape
Pipeline candidates such as Cyclo Therapeutics’ Trappsol Cyclo, Azafaros’ Nizubaglustat, Mandos’ Adrabetadex (VTS-270), and others are expected to drive the rise in NPC market size.
Cyclo Therapeutics’ Trappsol Cyclo is a specialized hydroxypropyl ß-cyclodextrin formulation that has shown encouraging results in multiple clinical studies for correcting cholesterol transport. By functioning as a substitute for the defective NPC1 protein, its cyclic structure facilitates the removal of trapped cholesterol from lysosomes, enabling proper cellular processing and clearance. It is currently being evaluated in clinical trials as a potential therapy for Niemann-Pick disease type C (NPC), a rare, progressive, and life-threatening genetic disorder.
Azafaros’ Nizubaglustat (formerly AZ-3102) is an orally administered, brain-penetrant azasugar with a distinctive dual mechanism of action, developed to treat rare lysosomal storage disorders with neurological involvement, including GM1 and GM2 gangliosidoses and NPC. Nizubaglustat works by modulating lipid metabolism to reduce the harmful buildup of “waste” lipids in lysosomes. By targeting this fundamental cellular dysfunction, it has the potential to act as a disease-modifying therapy for NPC, offering convenient at-home administration and improved quality of life for patients.
The anticipated launch of these emerging NPC therapies are poised to transform the NPC market landscape in the coming years. As these cutting-edge NPC therapies continue to mature and gain regulatory approval, they are expected to reshape the NPC market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for NPC, visit @ Niemann-Pick Disease Type C Medication
Recent Developments in the NPC Market
- In July 2025, the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending marketing authorisation for AQNEURSA to treat neurological manifestations of NPC.
- In July 2025, Azafaros announced the initiation of two global Phase III studies with Nizubaglustat in NPC and GM1/GM2 gangliosidoses, respectively.
- In March 2025, Rafael Holdings completed a merger with Cyclo Therapeutics following shareholder approvals.
- In February 2025, Cyclo Therapeutics presented preliminary data from the Phase III TransportNPC open-label sub-study at the 21st Annual WORLDSymposium 2025, showing that among NPC1 patients under 3 years old, 87% demonstrated stabilization or improvement on the CGI-C scale at 24 weeks and 86% at 48 weeks.
What is Niemann-Pick Disease Type C?
Niemann-Pick disease type C is a progressive and irreversible lysosomal lipid storage disorder that causes long-term, debilitating effects, affecting the viscera, nervous system, and mental health. The condition arises from impaired intracellular cholesterol trafficking, resulting in the buildup of unesterified cholesterol and glycosphingolipids in neurovisceral tissues. Most cases are due to mutations in the NPC1 gene, while some involve NPC2 mutations, both of which interfere with proper cholesterol transport within late endosomes and lysosomes. NPC also leads to a secondary decrease in ASM activity, connecting it to other forms of Niemann-Pick disease.
Niemann-Pick Disease Type C Epidemiology Segmentation
The NPC epidemiology section provides insights into the historical and current Niemann-Pick disease type C patient pool and forecasted trends for the leading markets. In 2024, the highest burden of NPC in the US was observed in the juvenile age group (6 to <15 years) with approximately 120 cases, followed by around 110 cases in the adolescent/adult-onset group (=15 years).
The Niemann-Pick disease type C market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets, segmented into:
- Prevalent Cases of NPC
- Total Diagnosed Prevalent Cases of NPC
- Age-specific Cases of NPC
- Gender-specific Cases of NPC
- Mutation-specific Cases of NPC
- Total Treated Cases of NPC
Download the report to understand Niemann-Pick disease type C management @ Niemann-Pick Disease Type C Treatment Options
| Niemann-Pick Disease Type C Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Niemann-Pick Disease Type C Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Niemann-Pick Disease Type C Market CAGR | 14.8% |
| Niemann-Pick Disease Type C Market Size | USD 60 Million |
| Key Niemann-Pick Disease Type C Companies | Cyclo Therapeutics, Azafaros, Mandos, IntraBio, Zevra Therapeutics, and others |
| Key Niemann-Pick Disease Type C Therapies | Trappsol Cyclo (hydroxypropyl-beta-cyclodextrin), Nizubaglustat (AZ-3102), Adrabetadex (VTS-270), AQNEURSA, MIPLYFFA, and others |
Scope of the Niemann-Pick Disease Type C Market Report
- Niemann-Pick Disease Type C Therapeutic Assessment: Niemann-Pick Disease Type C current marketed and emerging therapies
- Niemann-Pick Disease Type C Market Dynamics: Conjoint Analysis of Emerging Niemann-Pick Disease Type C Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Niemann-Pick Disease Type C Market Unmet Needs, KOL’s views, Analyst’s views, Niemann-Pick Disease Type C Market Access and Reimbursement
Discover more about NPC drugs in development @ Niemann-Pick Disease Type C Clinical Trials
Table of Contents
| 1 | Niemann-Pick Disease Type C Market Key Insights |
| 2 | Niemann-Pick Disease Type C Market Report Introduction |
| 3 | NPC Market Overview at a Glance |
| 3.1 | Market Share (%) Distribution of NPC by Therapies in 2024 in the 7MM |
| 3.2 | Market Share (%) Distribution of NPC by Therapies in 2034 in the 7MM |
| 4 | Epidemiology and Market Methodology |
| 5 | Executive Summary |
| 6 | Key Events |
| 7 | Disease Background and Overview |
| 7.1 | Introduction |
| 7.2 | Classification of NPD |
| 7.3 | Niemann-Pick Disease Type C Causes |
| 7.4 | Niemann-Pick Disease Type C Inheritance Patterns |
| 7.5 | Niemann-Pick Disease Type C Clinical Manifestations |
| 7.6 | Niemann-Pick Disease Type C Pathophysiology |
| 7.7 | Niemann-Pick Disease Type C Diagnosis |
| 8 | Niemann-Pick Disease Type C Treatment and Management |
| 9 | Epidemiology and Patient Population |
| 9.1 | Key Findings |
| 9.2 | Assumptions and Rationale |
| 9.3 | Total Diagnosed Prevalent Cases of NPC in the 7MM |
| 9.4 | The United States |
| 9.4.1 | Prevalent Cases of NPC in the United States |
| 9.4.2 | Total Diagnosed Prevalent Cases of NPC in the United States |
| 9.4.3 | Age-specific Cases of NPC in the United States |
| 9.4.4 | Gender-specific Cases of NPC in the United States |
| 9.4.5 | Mutation-specific Cases of NPC in the United States |
| 9.4.6 | Total Treated Cases of NPC in the United States |
| 9.5 | EU4 and the UK |
| 9.6 | Japan |
| 10 | Niemann-Pick Disease Type C Patient Journey |
| 11 | Marketed Niemann-Pick Disease Type C Therapies |
| 11.1 | Key Cross Competition |
| 11.2 | AQNEURSA (levacetylleucine): IntraBio |
| 11.2.1 | Product Description |
| 11.2.2 | Regulatory Milestones |
| 11.2.3 | Other Developmental Activities |
| 11.2.4 | Safety and efficacy |
| 11.2.5 | Analyst Views |
| 11.3 | MIPLYFFA (arimoclomol): Zevra Therapeutics (Formerly Known as KemPharm) |
| 12 | Emerging Niemann-Pick Disease Type C Therapies |
| 12.1 | Key Cross Competition |
| 12.2 | Trappsol Cyclo (Hydroxypropyl β-cyclodextrin): Cyclo Therapeutics |
| 12.2.1 | Product Description |
| 12.2.2 | Other Developmental Activities |
| 12.2.3 | Ongoing Clinical Development activity |
| 12.2.4 | Safety and Efficacy |
| 12.2.5 | Analyst Views |
| 12.3 | Nizubaglustat: Azafaros |
| 13 | NPC Market - Seven Major Market Analysis |
| 13.1 | Key Findings |
| 13.2 | Niemann-Pick Disease Type C Market Outlook |
| 13.3 | Conjoint Analysis |
| 13.4 | Key Niemann-Pick Disease Type C Market Forecast Assumptions |
| 13.5 | Total Market Size of NPC in the 7MM |
| 13.6 | Market Size of NPC by Therapies in the 7MM |
| 13.7 | Niemann-Pick Disease Type C Market Size in the United States |
| 13.7.1 | Total Market Size of NPC in the United States |
| 13.7.2 | Market Size of NPC by Therapies in the United States |
| 13.8 | Niemann-Pick Disease Type C Market Size in EU4 and the UK |
| 13.9 | Niemann-Pick Disease Type C Market Size in Japan |
| 14 | KOL Views on Niemann-Pick Disease Type C |
| 15 | Niemann-Pick Disease Type C Market SWOT Analysis |
| 16 | Niemann-Pick Disease Type C Market Unmet Needs |
| 17 | Niemann-Pick Disease Type C Market Access and Reimbursement |
| 17.1 | United States |
| 17.2 | EU4 and the UK |
| 17.3 | Japan |
| 17.4 | Reimbursement Scenario in NPC |
| 18 | Bibliography |
| 19 | Niemann-Pick Disease Type C Market Report Methodology |
Related Reports
Niemann-Pick Disease Type C Clinical Trial Analysis
Niemann-Pick Disease Type C Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key NPC companies, including Cyclo Therapeutics, Inc., Azafaros A.G., Cyclarity Therapeutics, Cyclolab, among others.
Niemann Pick Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Niemann Pick disease companies, including Azafaros A.G., Cyclo Therapeutics, Inc., ZevraDenmark, Genzyme, Mandos Health, Zevra Therapeutics, IntraBio, among others.
Niemann Pick Disease Clinical Trial Analysis
Niemann Pick Disease Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Niemann Pick disease companies, including Cyclo Therapeutics, Azafaros, IntraBio, among others.
Niemann Pick Disease Type A Market
Niemann Pick Disease Type A Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Niemann Pick disease type A companies, including Mandos LLC, Cyclo Therapeutics, Orphazyme, IntraBio Inc., Synaptogenix, among others.
Niemann Pick Disease Type A Clinical Trial Analysis
Niemann Pick Disease Type A Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Niemann Pick disease type A companies, including Mandos LLC, Cyclo Therapeutics, Orphazyme, IntraBio Inc., Synaptogenix, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
